Ionization Energy :
You are Quite familiar with the atom and you know atom is neutral because it contains equal number of Protons & Electrons. If we add an electron or remove an electron from it will disturb its neutrality of atom. And it become charged particle, called as ” ion “
It either develops an positive charged or negative charged ions.
Definition : ( Ionization Energy )
The term ionization Energy means the minimum energy required to remove the last Valence electron is called Ionization Energy.
Valence Electrons – Electrons Present in Outermost orbital of an atom
You all know all electrons are placed in orbit and sub-shells. So it have an address.
What makes the difference between those electrons ??
Well, Energy..!
The electron present closely to the nucleus will have an greater Attraction force of protons, so higher energy are required to remove those electrons. But in case of the electrons present far away from nucleus will have an lower Attraction force of protons, so minimum of energy are required to remove those electrons
The process is called as ionization.
The energy required to ionization is known as ionization Energy or ionization Enthalpy.
-

Nuclear Charge Balance
It is possible to remove more than one electron from the atom but you have to proceed step by step.
• The energy required to remove the electron from the Neutral Atom is Known as First ionization energy or ionization Enthalpy
• The energy required remove the electron from the Atom with one Positive Charge (A+) is known as Second ionization energy.
• The energy required to remove the electron from the atom with two positive charge (A++) is known as Third ionization energy.
 |
| Ionization Energy trend |
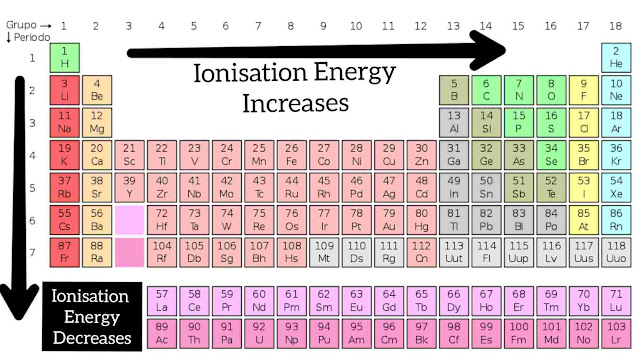
Ionization energy:- ( Across the Group )
When you go downward the group (column) the nuclear charge increases, but there is also an increase in the atomic size and orbits. That makes the valence electrons loosely attracted by the nucleus. so then we move from downwards on the group there is the decrease in the Ionization Energy.
The atom at the top of the group have the highest ionization energy whereas the lower one has lesser ionization Energy.
Ionization energy:- ( Across the Period )
When you go left to right in the period (row), there is an increase in nuclear charge but a decrease in atomic size. There is an increase in the number of protons makes the nuclear charge stronger. So it would be difficult to remove the electron from a smaller atom than from a larger atom. Because electrons in a smaller atom are bound tightly when compared with larger atoms. So the Ionization Energy increases in moving through the period
Alkali metals have the lowest ionization Energy
Noble gases have the highest ionization Energy
 |
| Trend Order |
Deviations in ionization Energy IE:-
But there is some deviations in the ionization energy trend due to the stability of Half filled & Full filled electronic configuration.
The deviations of Ionization Energy will be posted as Separate content.
Crack Chemistry – Do follow
Originally posted 2022-10-28 06:15:00.

