Benzene(C₆H₆) , the simplest aromatic hydrocarbon occupies the key position on the study of Chemistry of Aromatic compound.
This is one of the important topic in organic chemistry, In every exam at least one question is asked from this part.
Aromaticity is simply defined as behavior of benzene like compounds by other cyclic compounds
Aromatic compounds :-
The compound which behave like benzene are called Aromatic compound. An aromatic compound must satisfy the following conditions :-
• Cyclic and planer structure
• Equal bond length
• Chemical stability
• Ability to undergo substitution reactions
• Ability to sustain on induced diamagnetic ring current diastrophic
• Conjugated system of π-electrons with effective Delocalization
• All carbon present in the ring must be sp² – Hybridized
Huckel’s Rule of Aromaticity :-
An aromatic compound must contain a cyclic and planer structure with (4n+2)π electrons Delocalizing effectively throughout the plane of the molecule.
Thus, the Cyclic and planer compounds containing 2,6,10,14,18 etc., of π- electrons with effective Delocalization will have a aromatic character
Benzene – Aromatic character :-
(i). Benzene has a cyclic and planer structure with 6 Carbon atoms
(ii). It has an equal bond length of 1.39 A° between all carbon atoms which is neither a single bond 1.54 A° nor a double bond 1.34 A°
(iii). It has a resonance stabilization energy of 150.6 KJ/mole or 36 Kcal/mole
(iv). It has (4n+2)π electron where n=1 with effective Delocalization.
(v). It has a closed loop of 6-electrons called Aromatic Socket.
(vi). It undergoes substitution reactions rather than addition reaction
(vii). It has an ability to sustain an induced diamagnetic ring current and therefore it is diastrophic.
(viii). The PMR chemical shift of benzene protons appear around 7.8 – 8 value.
Aromatic compounds – Example :-
• Cyclopropenyl cation :-
 |
| Cyclopropenyl cation |
It is a cyclic and planer.
It has 2π electrons which are involved in the delocalizing through the Empty P-orbital of sp² hybrid carbon atom of carbocation in the ring. Therefore it is Aromatic.
• Cyclopropenone :-
 |
| Cyclopropenone |
It is a cyclic and planer Molecules with 2π electrons ,since the Electronegative Oxygen atom withdraws the electron towards itself, the resulting is resonance. The π – electrons are delocalized through the empty P-orbital of carbonium ion. Hence it is an Aromatic Compound.
• Cyclobutenyl di-cation :-
 |
| Cyclobutenyl di-cation |
It is cyclic and planer with 2π electrons which are delocalized through the empty P-orbitals of the two sp² hybridized carbon atom.
Anti-aromatic compounds :-
Anti-aromatic compounds must satisfy the following conditions,
• Cyclic and planer structure
• Conjugated system of (4n)π electrons with effective Delocalization.
• Sustain an induced paramagnetic ring current – Paratropic
• Equal bond length
• Less chemical stability
• Able to undergo substitution reactions
Example :-
• Cyclopropenyl anion :-
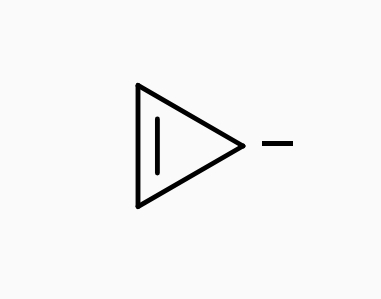 |
| Cyclopropenyl anion |
This is a cyclic and planer ion.
It has 4n – π electrons (n=1) including the two electrons of the negatively charged carbon atom.
These 4π electrons are involved in the effective Delocalization throughout the molecule.
It sustain an induced paramagnetic ring current.
Therefore it is an Anti-aromatic compound.
Non-aromatic compounds :-
Some compounds may have cyclic structure with conjugated double bonds but fail to have planer shape.
Such compounds do not have any aromatic character and they are called as Non – aromatic compounds.
Example :-
• Cyclopentadiene :-
 |
| Cyclopentadiene |
It is a cyclic and planer molecule, it has 4π electrons which does not obey the Huckel’s rules of Aromaticity due to presence of sp³ hybridized carbon atom that Molecule does not have any Delocalisation.so it is an Non – aromatic compounds
• Cycloheptatriene :-
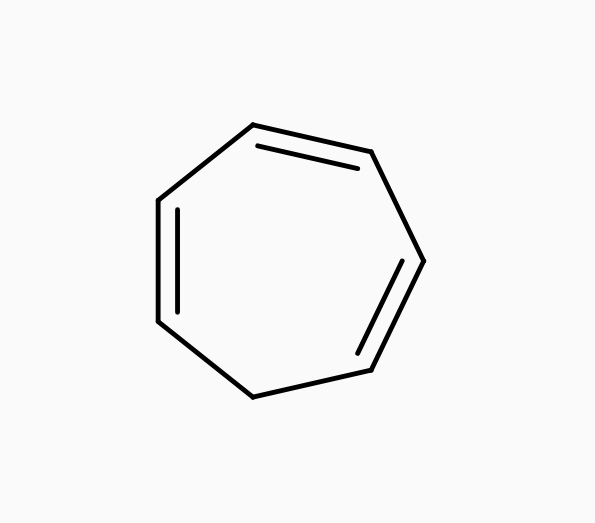 |
| Cycloheptatriene |
It is a cyclic and planer molecule, it has 6π electrons which does not obey the Huckel’s rules of Aromaticity due to presence of sp³ hybridized carbon atom that Molecule does not have any Delocalisation.so it is an Non – aromatic compounds
Conclusion :-
It must have cyclic and planer structure
All the carbon involved in ring must have sp² hybridized
Huckel’s rule of Aromaticity — (4n+2)π electrons
Huckel’s rule of Anti-Aromaticity — (4n)π electrons
***End****
I hope this article helps,
Crack Chemistry – Do follow
Originally posted 2022-10-29 07:55:00.


